Abstract
Introduction: Essential thrombocythemia (ET) is a myeloproliferative neoplasm (MPN) characterized by continued expansion of the megakaryocytic lineage in the bone marrow and peripheral blood, and by disease-associated symptoms such as headache, pruritus, concentration problems, and dizziness. Moreover, thromboembolic complications, progression to post-ET myelofibrosis, and acute leukemia are hallmarks of the disease and important clinical endpoints to prevent. Cytoreductive treatment with hydroxyurea (HU) or anagrelide is approved for the treatment of patients (pts) with ET. However, the majority of pts continue to suffer from ET-associated symptoms despite treatment. Ruxolitinib (RUX) is approved for myelofibrosis and HU-intolerant/-resistant polycythemia vera (PV). In clinical trials, RUX demonstrated symptom relief in HU-intolerant/-resistant ET but failed to show superiority over second-line best available therapy (BAT) in inducing complete or partial response. However, RUX vs. BAT therapy has not been investigated in pts with untreated or non-intolerant/-resistant ET. We hypothesized that RUX may have higher efficacy in such earlier-line ET pts.
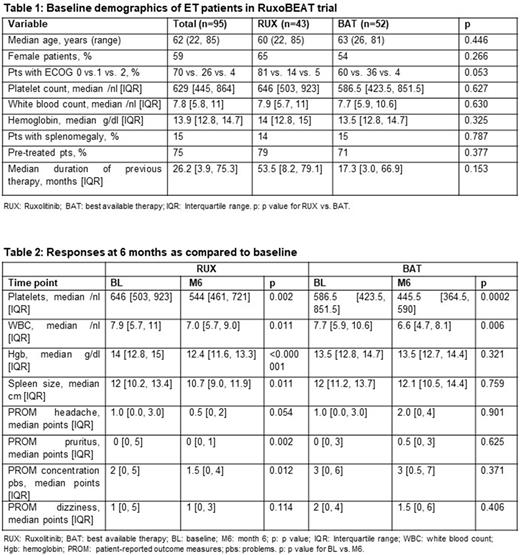
Methods: The clinical trial entitled "Ruxolitinib versus Best Available Therapy in pts with high-risk PV or high-risk ET" (RuxoBEAT; NCT02577926) is a multicenter, open-label, two-arm phase-IIb trial with a target population of 190 pts each with PV and ET. Crossover from BAT to RUX is possible in eligible patients after 6 months. Patients with ET in the RUX arm receive a starting dose of 10 mg BID and may increase their dose up to 25 mg BID. The primary endpoint is the clinicohematologic complete response (CR) rate at month 6, as defined by Barosi et al (Blood 2009), but using a strict score of zero for the four symptoms by patient-reported outcome measures (PROM) (Table 2) and an adjusted level alpha = 0.005 at a power of 80%. Secondary endpoints include overall response rate (CR+PR), changes in blood counts, spleen size, or PROM, using p values descriptively. This is a pre-specified interim analysis of 95 ET pts randomized 1:1 to RUX vs. BAT. ET pts are allowed to have had previous ET-directed therapy or no therapy. Differences between baseline and end of month 6 were calculated using Fisher´s exact test (for binary variables) or the Wilcoxon signed-rank test (for ordered or continuous variables).
Results: 95 pts (75% were pre-treated) were included in the intention-to-treat set (n=43 randomized to RUX and n=52 randomized to BAT) and analyzed at the 6-month time point. Relevant baseline characteristics are listed in Table 1. In patients on RUX or BAT, CR rate was 0 and 1.9% (p=1.0) and CR+PR rate was 58.1% and 76.9% (p=0.075), respectively. At month 6, there was a significant difference between RUX and BAT in the median number of platelets (544/nl vs. 445.5/nl, p=0.013), median hemoglobin values (12.4g/dl vs. 13.5g/dl, p=0.001), and median spleen size (10.7cm vs. 12.1cm, p=0.002), but not in the median number of white blood cells (WBC) (7.0/nl vs. 6.6/nl, p=0.177). However, pts randomized to RUX reported, in median, significantly lower PROM points than BAT for headache (0.5 vs. 2.0, p=0.034) and concentration problems (1.5 vs. 3.0, p=0.007) and a trend for improvement for pruritus (0.0 vs. 0.5, p=0.09), but no significant difference for dizziness (1.0 vs. 1.5, p=0.162).
At month 6, compared to baseline, RUX significantly reduced median platelet count, WBC, hemoglobin, spleen size, PROM for pruritus and concentration problems, but not headache or dizziness (Table 2). On the other hand, BAT significantly reduced median platelet count and WBC, but not hemoglobin, spleen size, or any of the four PROM symptoms (Table 2).
Safety analysis revealed 340 adverse events (AE) in both assigned treatment groups, including 143 AE in the RUX and 197 AE in the BAT group (5.6% and 11% grade ≥3, resp.). There was no significant difference in the percentage of grade ≥3 AE between both groups (p=0.083). There were no consistent types of grade ≥3 AE in either of the two arms.
Conclusion: In this pre-specified interim analysis, treatment with ruxolitinib was not superior over BAT to induce complete response (using strict criteria for symptoms) in ET pts that were either untreated or not intolerant/resistant to prior therapy. However, RUX was more effective in reducing ET-associated spleen size and symptoms, such as headache and concentration problems. The RuxoBEAT trial is ongoing.
Disclosures
Koschmieder:RWTH Aachen University: Patents & Royalties: BET Inhibitor; Sierra Oncology: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel support; Karthos: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel support; Imago Biosciences: Membership on an entity's Board of Directors or advisory committees, Other: Travel support; Alexion: Other: Travel support; AbbVie: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel support; PharmaEssentia: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel support; Bayer: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel support; Roche: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel support; CTI Biopharma: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel support; Celgene: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel support; Ariad: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel support; Bristol Myers Squibb: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel support; Incyte: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel support; Pfizer: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel support; AOP Pharma: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel support, Research Funding; Janssen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel support, Research Funding; Novartis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel support, Research Funding; Geron: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel support, Research Funding. Isfort:Pfizer: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: e.g. travel support; Incyte: Honoraria, Membership on an entity's Board of Directors or advisory committees; Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: e.g. travel support; BMS: Honoraria; Alexion: Other: e.g. travel support; Mundipharma: Other: e.g. travel support; Roche: Other: e.g. travel support; Hexal: Other: e.g. travel support. Heidel:Novartis: Consultancy, Honoraria, Research Funding. Hochhaus:Bristol Myers Squibb: Research Funding; Pfizer: Research Funding; Novartis: Research Funding; Incyte: Research Funding. Becker:Novartis: Honoraria. Jost:Ariad: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: e.g. travel support, Research Funding; Abbvie: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: e.g. travel support, Research Funding; Bayer: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: e.g. travel support; Boehringer: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: e.g. travel support, Research Funding; Novartis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: e.g. travel support, Research Funding; Pfizer: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: e.g. travel support; Servier: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: e.g. travel support; Roche: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: e.g. travel support, Research Funding; BMS/Celgene: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: e.g. travel support, Research Funding; Pierre Fabre: Other: e.g. travel support; Janssen (Johnson & Johnson): Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: e.g. travel support; MSD: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: e.g. travel support, Research Funding. Schafhausen:Alexion: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: e.g. travel support; AOP Orphan: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: e.g. travel support; Blueprint Medicines: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: e.g. travel support; BMS/Celgene: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: e.g. travel support; Merck Serono: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: e.g. travel support; MSD: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: e.g. travel support; Novartis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: e.g. travel support; Pfizer: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: e.g. travel support; Roche: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: e.g. travel support; Sobi: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: e.g. travel support. Haenel:Takeda: Consultancy, Honoraria; GSK: Consultancy, Honoraria; Gilead: Consultancy, Honoraria; Celgene: Consultancy, Honoraria; Bayer: Consultancy, Honoraria; Amgen: Consultancy, Honoraria; Pfizer: Honoraria; Novartis: Consultancy, Honoraria; Roche: Consultancy, Honoraria; JAZZ: Consultancy, Honoraria. Göthert:Proteros Biostructures: Honoraria; zr pharma&: Honoraria; BMS: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: travel support; Ariad: Honoraria, Membership on an entity's Board of Directors or advisory committees; Pfizer: Honoraria, Membership on an entity's Board of Directors or advisory committees; Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel support; AOP Pharma: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel support; Incyte: Membership on an entity's Board of Directors or advisory committees, Other: Travel support; Abbvie: Membership on an entity's Board of Directors or advisory committees; CTI Biopharma: Membership on an entity's Board of Directors or advisory committees; Imago Bioscience: Membership on an entity's Board of Directors or advisory committees. Teichmann:Astellas: Consultancy; BMS: Consultancy, Honoraria; Pfizer: Consultancy; Boehringer-Ingelheim: Honoraria; AOP: Honoraria; Sobi: Consultancy, Honoraria; BeiGene: Other; Gilead: Other: n. Sockel:Active Biotech: Research Funding; Gilead: Honoraria; SOBI: Honoraria; BMS: Consultancy, Honoraria; Novartis: Consultancy, Honoraria. Radsak:Takada: Honoraria; SOBI: Honoraria; BMS: Honoraria; Novartis: Honoraria; Cogent: Consultancy; CORAT: Consultancy; AOP: Honoraria; Daiichi Sankyo: Honoraria. Reiter:Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Blueprint Medicines: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Incyte: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Abbvie: Membership on an entity's Board of Directors or advisory committees, Research Funding; AOP: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; GSK: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding. Döhner:AbbVie: Honoraria, Membership on an entity's Board of Directors or advisory committees; Jazz: Honoraria, Membership on an entity's Board of Directors or advisory committees; BMS/Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Agios: Research Funding; Astellas: Research Funding; Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Kronos: Research Funding. Jaekel:Novartis: Honoraria, Other: Payment. Stegelmann:Novartis: Consultancy, Honoraria; Incyte: Consultancy, Honoraria; Pfizer: Consultancy, Honoraria; BMS/Celgene: Consultancy, Honoraria; AOP Pharma: Consultancy, Honoraria. Gezer:GSK: Honoraria, Membership on an entity's Board of Directors or advisory committees; Takeda: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel support; Janssen/Cilag: Other: Travel support; BMS: Honoraria; Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel support; Amgen: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel support. Frank:RWTH Aachen University: Other: Funding from the sponsor of the study (RWTH Aachen University) for statistical planning and analysis. Hellmich:RWTH Aachen University: Other: Funding from the sponsor of the study (RWTH Aachen University) for statistical planning and analysis. Brümmendorf:Novartis: Consultancy, Honoraria, Other: travel grant, Research Funding; Pfizer: Consultancy, Honoraria, Other: travel support, Research Funding; Janssen: Consultancy, Other: travel support; Merck: Consultancy, Other: travel support.
OffLabel Disclosure:
Comparison of ruxolitinib vs. best available therapy in patients with high-risk essential thrombocythemia (ET). Ruxolitinib is currently not approved for the treatment of patients with ET or untreated PV.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal